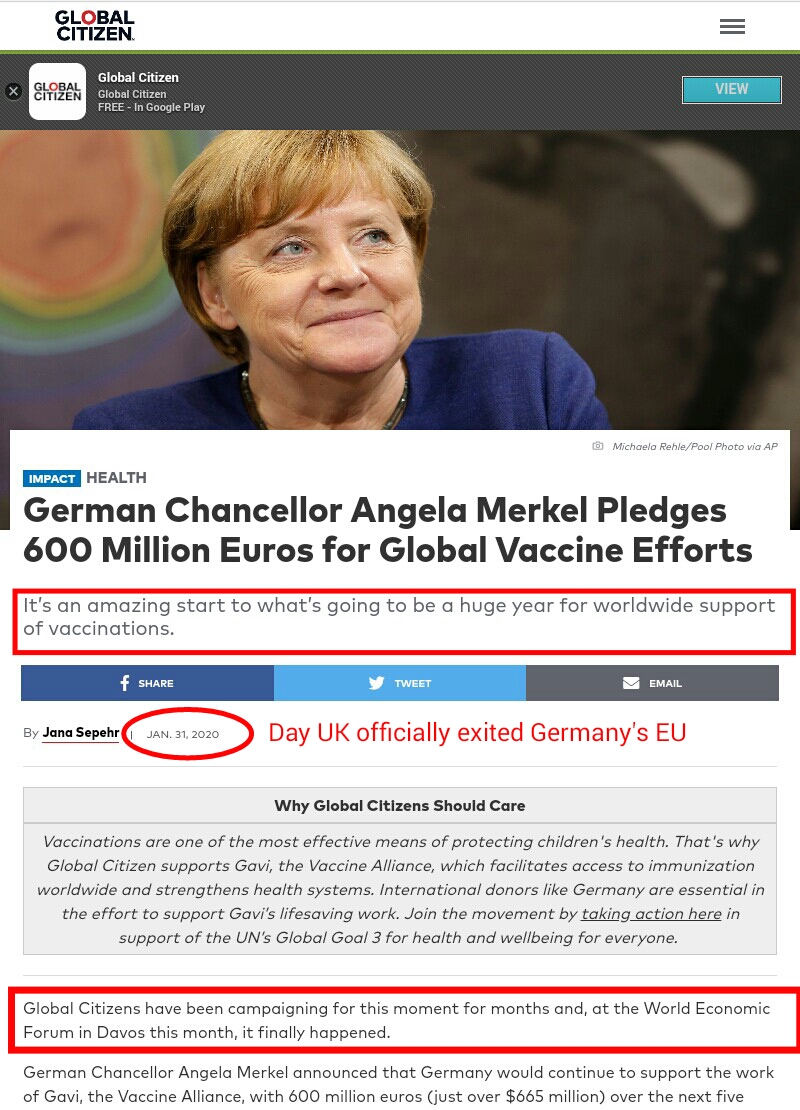
Hydrogen fuel you can easily produce yourself using electrolysis of water
Getting Off Oil, World news Sunday, May 26th, 2013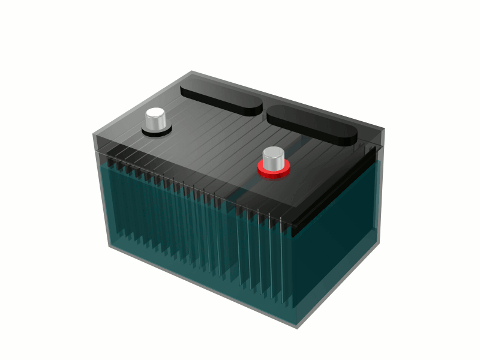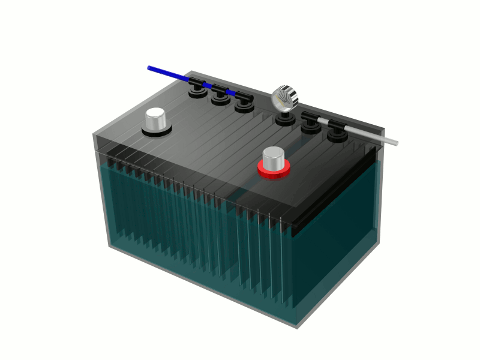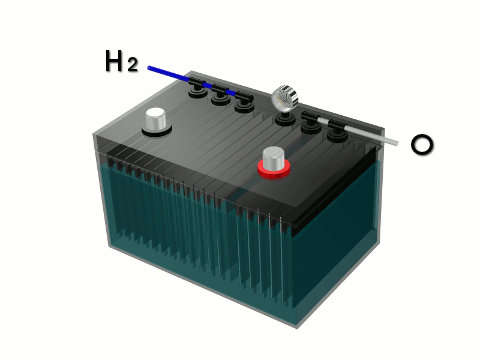
A hydrogen fuel cell is nothing more than a redesigned car battery without the battery acid
Free natural gas is available for everyone living near water. The natural gas known as hydrogen can be generated from water – tap water, sea water, drain water, any water. Water is also known as H2O or Hydrogen and Oxygen. Water can easily be split (fissioned) into zero emission hydrogen fuel using a simple method called electrolysis.
The technology of using water as a hydrogen fuel isn’t new as claimed today by most major newspapers and cable networks. The technology was patented and demonstrated by Stanley Myers in a 1990 US Patent 5149407 – Process and apparatus for the production of fuel gas and the enhanced release of thermal energy from such gas – “Water molecules are broken down into hydrogen and oxygen gas atoms in a capacitive cell by a polarization and resonance process dependent upon the dielectric properties of water and water molecules. The gas atoms are thereafter ionized or otherwise energized and thermally combusted to release a degree of energy greater than that of combustion of the gas in ambient air.”
What Stanley Myers did was use electrolysis of water to separate the bond between the hydrogen and oxygen gas in water (H2O) and produced a highly combustible and clean burning hydrogen gas fuel. Electrolysis of water is the splitting of water (H2O) into oxygen (O) and hydrogen gas (H2) due to an electric current being passed through the water. An electrical power source (solar panel, car battery) is connected to two electrodes, or two plates (typically made from some inert metal such as platinum or stainless steel) which are placed in the water. Hydrogen gas will rise from the cathode (the negatively charged electrode, where electrons enter the water), and oxygen gas will rise at the anode (the positively charged electrode).
Does the accompanying article image look familiar to you? It should. It is a CAD (computer aided design) representation of a mass produced auto part that can be found in the engine compartment of every gas combustion engine vehicle ever made. It is a car battery. Car batteries have more than enough stored energy to perform electrolysis of water as you drive. A car battery can also be used as the hydrogen fuel cell – where water is split into a hydrogen fuel gas.
Most car batteries are lead-acid batteries. Each cell has an electrolytic solution of sulfuric acid (H2SO4) and water. Newer vehicles have more sophisticated batteries of the nickel-metal hydride variety. The electrolyte in them is generally potassium hydroxide (KOH) or a similar alkaline hydroxide. You can order a car battery with no electrolytic solution or any liquid whatsoever. A new dry car battery can be easily made into a hydrogen fuel cell. It already has everything you need built in to start the electrolysis of water and start producing your own hydrogen fuel for your car or truck. It has a positive (anode) and negative (cathode) posts needed to apply an electrical current and split water into hydrogen and oxygen gas using electrolysis of water. It also has several plates already installed within it from which hydrogen gas bubbles will form off of. You need only to add an electrolyte other than the usual acid in order to make the hydrogen fuel cell more efficient – produce enough hydrogen gas to run you vehicle’s engine. Sea salt is a very good electrolyte but it is very corrosive and can produce brown gas instead of clean burning hydrogen gas. Baking soda is a very good electrolyte.
In electrolysis of water a very small electrical current is passed between two electrodes submerged in water. Electrolysis of water will begin with a minimum of 1.2 volts and will increase in rate as the voltage is increased. Sources of electrical current that can be used in the electrolysis of water – car battery (12 volts), solar panel (5-15 watts), flashlight battery (1.5 volts), cell phone battery (3.7 volts). 12 volts (just 10% or 1/10 the electrical power needed to power the toxic fluorescent lights which requires 120 volts to operate) would produce more than enough hydrogen fuel to power a gas combustion engine like a portable electrical power generator. The hydrogen gas from water can also be used to fuel gas stoves, gas fireplaces, gas barbecues and gas home heating furnaces. Hydrogen gas from water produces no toxic carbon emissions. It is a carbon free, environmentally friendly and unmetered fuel.

Don’t be fooled by news stories claiming a new hydrogen fuel source has been developed by the auto makers. Hydrogen fuel has been available to mankind since the stone ages. You just weren’t told it existed because they haven’t figured out how to charge you for something that you can produce yourself.
Videos I made and own demonstrating how electrolysis of water can easily split water into a hydrogen fuel and oxygen:
FuelReducer H2O – Water for Fuel Project
You can download and share the videos as often as you like as there is no copyright infringement as I made both the hydrogen fuel cells shown and the videos. The videos are posted as public domain. They were made to educate the public as to how easy it is to produce hydrogen fuel from plain ordinary tap water, river water, stream water, rain water, and sea water. No toxic chemicals were added like sulfuric acid that is used to produce hydrogen sulfide (H2S) that gas companies are calling shale gas. H2S is a colorless gas with the characteristic foul odor of rotten eggs; it is heavier than air, very poisonous, corrosive, flammable and explosive. Hydrogen sulfide and oxygen burns as a blue flame to form sulfur dioxide (SO2) and water. It is the H2S blue flame that shale gas companies and government are fraudulently marketing as shale gas. Shale gas doesn’t exist. It is snake oil marketing. A rebranding of the very poisonous H2S as a natural gas.
Hydrogen sulfide is considered a broad-spectrum poison, meaning that it can poison several different systems in the body, although the nervous system is most affected. The toxicity of H2S is comparable with that of hydrogen cyanide. It forms a complex bond with iron in the mitochondrial cytochrome enzymes, thus preventing cellular respiration.
When hydrogen sulfide (fabricated shale gas) and oxygen burns it forms sulfur dioxide (SO2) and water – a.k.a. acid rain. Sulfur dioxide is a major air pollutant and has significant impacts upon human health. In addition the concentration of sulfur dioxide in the atmosphere can influence the habitat suitability for plant communities as well as animal life. Sulfur dioxide emissions are a precursor to acid rain and atmospheric particulates.
Short URL: https://presscore.ca/news/?p=8060

 The Halifax International Security Forum was founded in 2009 as a propaganda program within the German Marshall Fund (founded June 5, 1972 by West German Chancellor Willy Brandt) by the Crown in Canada using Crown Corp ACOA & DND funds. The Halifax International Security Forum is a front that is used to recruit top US, UK and Canadian gov and military officials as double agents for Canada's WWI, WWII enemy and wage new Vatican Germany Cold War.
High Treason: s.46 (1) Every one commits high treason who, in Canada (c) assists an enemy at war with Canada, ..., whether or not a state of war exists". Every one who, in Canada assists Canada's enemies wage "piecemeal WWIII" Cold War by organizing, funding and participating in the Germany government politically and militarily benefitting / lead Halifax International Security Forum is committing high treason.
The Halifax International Security Forum was founded in 2009 as a propaganda program within the German Marshall Fund (founded June 5, 1972 by West German Chancellor Willy Brandt) by the Crown in Canada using Crown Corp ACOA & DND funds. The Halifax International Security Forum is a front that is used to recruit top US, UK and Canadian gov and military officials as double agents for Canada's WWI, WWII enemy and wage new Vatican Germany Cold War.
High Treason: s.46 (1) Every one commits high treason who, in Canada (c) assists an enemy at war with Canada, ..., whether or not a state of war exists". Every one who, in Canada assists Canada's enemies wage "piecemeal WWIII" Cold War by organizing, funding and participating in the Germany government politically and militarily benefitting / lead Halifax International Security Forum is committing high treason.
 Please take a moment to sign a petition to
Please take a moment to sign a petition to 







































 1917 Code of Canon Law, Canon 185 invalidates (voids) all papacies since October 26, 1958 due to the fact Cardinal Giuseppe Siri was elected Pope on the Third ballot on Oct 26 1958 but the new Pope Gregory XVII was illegally prevented from assuming the office. A Pope was elected on October 26, 1958. Thousands of people witnessed a new Pope being elected by seeing white smoke and millions were informed by Vatican radio broadcasts beginning at 6:00 PM Rome time on October 26, 1958. The papacy of Francis, Benedict, John Paul II, John Paul I, Paul VI, John XXIII and any and all of their respective doctrines, bulls, letter patents and the Second Vatican Council are all invalidated (having no force, binding power, or validity) by Canon 185 because the 1958 conclave of cardinals elected Cardinal Giuseppe Siri Pope on Oct 26 1958. Cardinal Giuseppe Siri accepted the papacy by taking the name Pope Gregory XVII but was illegally prevented from assuming his elected office.. According to Canon 185 Cardinal Angelo Giuseppe Roncalli illegally assumed the papacy 2 days later by fraud and grave fear, unjustly inflicted against Cardinal Giuseppe Siri who was lawfully elected Pope Gregory XVII. Because no Pope has been lawfully elected since October 26, 1958 the Holy See (la Santa Sede/Seat) remains vacant.
1917 Code of Canon Law, Canon 185 invalidates (voids) all papacies since October 26, 1958 due to the fact Cardinal Giuseppe Siri was elected Pope on the Third ballot on Oct 26 1958 but the new Pope Gregory XVII was illegally prevented from assuming the office. A Pope was elected on October 26, 1958. Thousands of people witnessed a new Pope being elected by seeing white smoke and millions were informed by Vatican radio broadcasts beginning at 6:00 PM Rome time on October 26, 1958. The papacy of Francis, Benedict, John Paul II, John Paul I, Paul VI, John XXIII and any and all of their respective doctrines, bulls, letter patents and the Second Vatican Council are all invalidated (having no force, binding power, or validity) by Canon 185 because the 1958 conclave of cardinals elected Cardinal Giuseppe Siri Pope on Oct 26 1958. Cardinal Giuseppe Siri accepted the papacy by taking the name Pope Gregory XVII but was illegally prevented from assuming his elected office.. According to Canon 185 Cardinal Angelo Giuseppe Roncalli illegally assumed the papacy 2 days later by fraud and grave fear, unjustly inflicted against Cardinal Giuseppe Siri who was lawfully elected Pope Gregory XVII. Because no Pope has been lawfully elected since October 26, 1958 the Holy See (la Santa Sede/Seat) remains vacant.
 Hold the Crown (alias for temporal authority of the reigning Pope), the Crown appointed Governor General of Canada David Lloyd Johnston, the Crown's Prime Minister (servant) Stephen Joseph Harper, the Crown's Minister of Justice and Attorney General Peter Gordon MacKay and the Crown's traitorous military RCMP force, accountable for their crimes of treason and high treason against Canada and acts preparatory thereto. The indictment charges that they, on and thereafter the 22nd day of October in the year 2014, at Parliament in the City of Ottawa in the Region of Ontario did, use force and violence, via the staged false flag Exercise Determined Dragon 14, for the purpose of overthrowing and besieging the government of Canada contrary to Section 46 of the Criminal Code. In a society governed by the rule of law, the government and its officials and agents are subject to and held accountable under the law. Sign the online
Hold the Crown (alias for temporal authority of the reigning Pope), the Crown appointed Governor General of Canada David Lloyd Johnston, the Crown's Prime Minister (servant) Stephen Joseph Harper, the Crown's Minister of Justice and Attorney General Peter Gordon MacKay and the Crown's traitorous military RCMP force, accountable for their crimes of treason and high treason against Canada and acts preparatory thereto. The indictment charges that they, on and thereafter the 22nd day of October in the year 2014, at Parliament in the City of Ottawa in the Region of Ontario did, use force and violence, via the staged false flag Exercise Determined Dragon 14, for the purpose of overthrowing and besieging the government of Canada contrary to Section 46 of the Criminal Code. In a society governed by the rule of law, the government and its officials and agents are subject to and held accountable under the law. Sign the online  Two of the most obvious signs of a dictatorship in Canada is traitorous Stephen Harper flying around in a "military aircraft" and using Canadian Special Forces "military" personnel from JTF2 and personnel from the Crown's traitorous martial law "military" RCMP force as his personal bodyguards.
Two of the most obvious signs of a dictatorship in Canada is traitorous Stephen Harper flying around in a "military aircraft" and using Canadian Special Forces "military" personnel from JTF2 and personnel from the Crown's traitorous martial law "military" RCMP force as his personal bodyguards.





































Every fuel cell now being designed is just an elaborate redesign of the car battery. Instead of supplying stored electrical energy for your vehicle’s electrical system the battery fuel cell will supply hydrogen gas fuel from water for the car’s “gas” combustion engine.